What is the BASIS KIDS Study?
The BASIS KIDS Study is testing a study drug for boys with either hemophilia A or B. The study will look at the safety of the study drug and what it does inside the body. Researchers want to see if this study drug can prevent bleeds that commonly happen in people with hemophilia.
The study drug is not factor replacement therapy. It blocks a substance naturally found in the body that stops the clotting process. By doing this, the study drug may lead to an increase in clotting activity.
Get to know Charlie and his mom, Hannah, as they talk about their experience with hemophilia and participation in clinical trials.

Who can participate?
To be considered for the study, your child must:
- Be a male younger than 18 years of age
- Have severe hemophilia A or moderately severe to severe hemophilia B, with or without inhibitors
- Meet additional requirements, as determined by a screening process
- Some people develop inhibitors to replacement factor therapy, which prevents it from working and forming a clot. This makes it more difficult to stop a bleed. The study drug being tested is not affected by inhibitors.
What happensduring the study?
Your child will be in the study for approximately 14 months. Throughout the study, your child will have on-site visits and phone calls with the study team to check on his health.
Once your child completes the study, he may have the option to continue taking the study drug for an extended period of time as part of a separate study. You can discuss this with your child’s study doctor if you are interested.
Permission
The study doctor will first review the Informed Consent Form with you, which contains all the details of the study. Take all the time you need to think it over and ask any questions you and your child have. If you agree to have your child join, you will give your permission by signing the form. Your child may also sign a similar form called the Assent Form noting that he agrees to take part in the study.
Screening
To see if your child qualifies for the study, the study doctor will ask questions about your child’s health, medical history, and medicines that he takes. Your child will also have a physical exam and some tests, including blood and urine tests. The screening period is approximately one month
Study Treatment
All children who qualify for the study will receive the study drug for 12 months. During this time, your child will also have about 9 on-site visits and 5 phone calls with the study team to check on his health. Some of these visits may take place at your home if you prefer and if local regulations allow it.
Follow-Up
If your child chooses not to continue taking the study drug as part of the separate study, the study doctor will contact you to check on your child’s health 30 days after his final dose of study drug.
How is the study drug given?
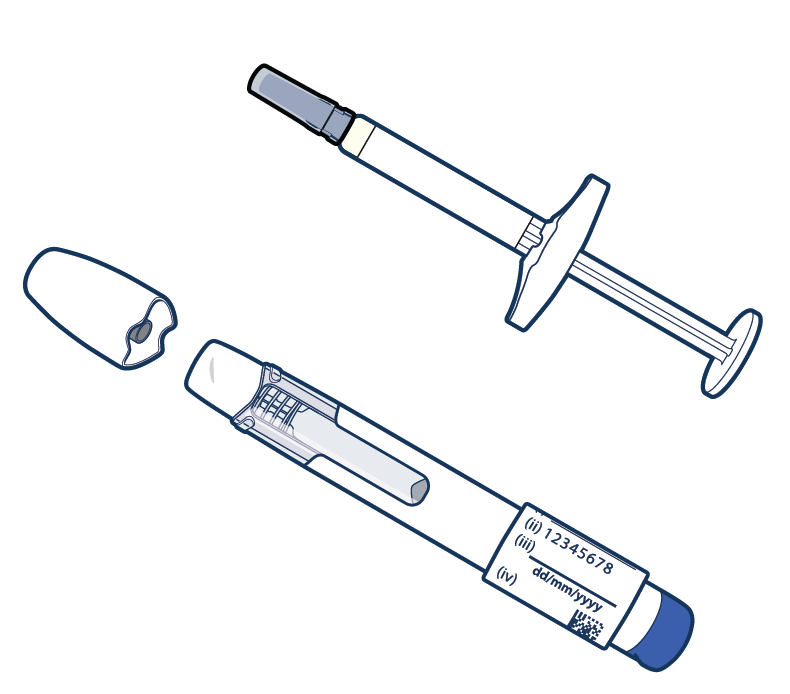
Unlike factor replacement therapy, which is given by regular infusions (slowly into the vein), the study drug is given as an injection (shot) into the fatty layer directly below the skin once a week.
The first injection will be given at the study site. The other doses will be given at home, or at the site if you prefer.
- Note: Where your child receives the injection may depend on if he is using a pre-filled syringe or pen.
- The location will also depend on if your child is giving himself the injection or if a caregiver or study doctor is administering the injection.
What types of tests will my child have at study visits?
At study visits, your child will have tests to check his health. Below are some tests that your child will have. He will not have all the tests at every visit.

Vital Signs
Physical Exam
Joint Check

Blood Tests

Urine Test
Heart Activity (ECG)

Questionnaires
Is there anything else we will need to do?
You will be given a handheld electronic device like a smartphone to collect information during the study, including:
- Any bleeds your child has
- Any medicines used to treat your child’s bleeds
- When your child receives his study drug injection
Find a Study Site
Below are a list of study centers in your area:

